
Ravi Elangovan’s decision to leave a cushy research job in Florence and join IIT-Delhi’s faculty in 2010 was driven mostly by personal reasons. “I always wanted to come back to India,” he said, as he settled down for a Skype interview on a Sunday morning to discuss the project that is validating his decision.
At the time, his father wasn’t keeping well and he wanted to live close by. But also, there was a desire to make a difference in his home country. “You so often hear India is the global capital of diseases like TB and Malaria. We need to solve our problems. Find low-cost, relevant solutions,” he says.
In a small yet significant way, Elangovan is helping make that happen. Backed by the ministry of HRD, part-funded by the government, supported by inter-disciplinary teams from IIT and guided by veterans from the medical sciences including those from Jamia Hamdard University and National Institute of Pathology, two low-cost, low-maintenance diagnostic tech tools are in the works that could potentially help diagnose tuberculosis cheaper, faster and better even in remote locations.
Tests are underway and regulatory approvals are pending, but there is excitement among Elangovan’s partners and supporters in the project, as initial test data is encouraging.
“Until we get sufficient validation I can’t comment on it. But what I can say is these projects are very important. We don’t have Indian diagnostic solutions. We need to develop them,” says Renu Swarup, secretary, Department of Biotechnology (DBT).
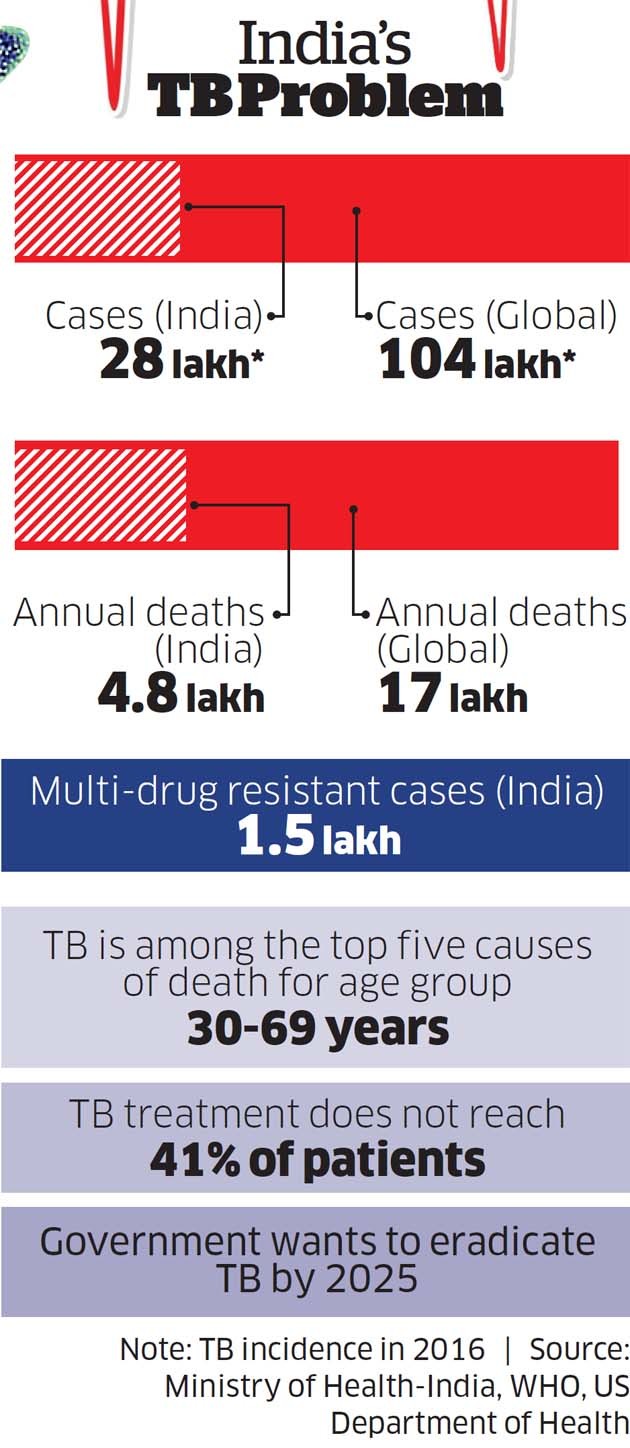
Even though these are early days, experts have welcomed the development. “SeeTB (see box) development is welcome. A low-cost fluorescent microscope will be useful in scaling up diagnostics rapidly,” says WHO India country office when reached for comments.
On iMC2 (see box), it said, “it is useful to have a low-cost, battery operated closed system point of care for TB”. But with the details not available, it said, it would be too early to comment. The project is entering crucial phase now.
Clinical validation is ongoing. Approvals from the Drug Controller General of India will be required. Elangovan, his motley team and their startup Valetude Primus Healthcare is readying to manufacture and commercially launch the product in the market next year. Competitive landscape is being scanned and the scenario looks daunting with over 10 such products from overseas and in India looking to tap the market.
“Innovations in medical devices face many death valleys,” Elangovan says. He is facing many now, but with hope intact. “We will push it and make it happen.” Success or failure, for multiple reasons Elangovan’s journey holds wider significance for India’s science and technology ecosystem and also how it tackles its healthcare challenges.
Traditionally, India has imported technology, which are costly, rather than build its own. From the IT services to BPO industry, our businesses have often been premised on labour and cost arbitrage rather than innovation. PhD and research in our universities— key ingredients to creating innovation— has been relatively weak, poorly paid and considered low brow in a nation that grooms the largest numbers of doctors and engineers in the world. Not surprisingly, the brightest of them join the corporate world or flee to the labs in the US and Europe.
It doesn’t help that India’s research outfits and universities often work in silos, where inter-institutional and inter disciplinary collaboration in research — critical for building successful applications — is poor.
“In the digital era where innovation holds key to a nation’s economic well-being, this is something crucial for India to get right,” says Varun Aggarwal, cofounder of Aspiring Minds and author of Leading Science and Technology: India Next, a book that deep-dives into India’s science and tech landscape. Research papers, citations and patents filed are some of the key yardsticks to vet a country’s innovation strength.
India does not fare too well—it produces a quarter of research papers that China (ranked second after the US) produces. Our research influence—measured by citations —is 3.39 as against 7.15 of the US and 4.29 of China.
In highly cited papers between 2012-2016, India had 989, US 15,000 and China 7,213. “Scientific research is a national necessity for our economic growth in the 21st century,” Aggarwal says.
It is for all the above reasons that Elangovan’s journey — from Italy to India, his forging inter-disciplinary collaborations to innovate and now trying to take to market the TB diagnostic platform — is an important story to tell.
In 2010, when Elangovan joined IIT-Delhi as a faculty member in the department of biophysics, he needed a single molecule microscope for his lab work. Costing upwards of Rs 1.5 crore, typically the lab would have imported them. “I wanted to build our own here,” he says. Buying off-the-shelf technology is quick, easy but costly and without any technology ownership. “India can learn a lot from research landscape in Italy. I was in an instrument-making lab.
Making world-class equipment didn’t need lot of money but offered enormous learning curve and exponential benefits,” he says. It took Elangovan two years and around Rs 30 lakh to build his own molecular microscope.
“Being at IIT has a huge advantage. You have so much expertise from different fields like physics, electronics, mechanical, biosciences inside the same campus,” he says. As he built, conversations with other experts helped. For example, from a senior faculty in the Physics MTech Lab he learnt about miniaturising expensive opto-electronic systems.
People from other streams joined on the way. In 2013, just when Elangovan got a Rs 85 lakh grant from the DBT for the project, he met Saurabh Singh and Vikas Pandey, IIT (Delhi) alumni in corporate jobs, earning well but thoroughly bored.
“From the biochemical field, we wanted to work in an area that we specialised in. In companies, we were doing nothing that related to our technical background,” says Pandey. The duo were looking for entrepreneurial opportunities and quit their well-paying ( Rs 1 lakh plus) corporate jobs to join as PhD scholars on a monthly Rs 25,000 stipend to work on the project.
A big boost came from Prof Sayed E Hasnain, vice-chancellor of Jamia Hamdard University and also a faculty member at IIT Delhi. Hasnain leads a centre of excellence on TB and is deeply engaged in the pursuit of research, diagnosis and drug solutions for TB. “It was a chance conversation with Ravi. I got to know about his single molecular microscope and suggested it could be used to develop platforms to diagnose bugs,” he says. That’s how the TB project germinated.
Hasnain’s wife Prof Nasreen Z Ehtesham, also acting director, National Institute of Pathology, also joined in. “We understand the biology of the bug. We work on vaccines and drugs. That’s where we stopped. We never worked with engineers and they were never with us. Proximity, trust all that has helped us work on this project together,” she says.
The IIT support, government grants, awards and recognition all came handy to strengthen the project and boost morale at different stages. In 2013, DBT sanctioned Rs 85 lakh. In 2017, the TB project was allocated Rs 2.2 crore as part of the Ministry of HRD’s IMPRINT programme where innovators must work closely with companies to commercialise innovations and take it to the market. In 2016, their project won Pfizer IITD Innovation award and the UK’s Longitude Prize Discovery Award. In 2018, SeeTB got the Gandhian Young Technology Innovation award. “These recognitions helped us gain traction here,” says Hasnain.
Bumpy Road Ahead
There have been many hurdles on the way, that Elangovan calls death valleys for medical devices innovation. Developing prototypes and then refining it for large-scale manufacturing is a difficult journey in India. “In China, there are manufacturing clusters where you go with a prototype and get the job done within days if not hours,” Elangovan says. Having a prototype manufacturing cluster with everything under one roof, like an SEZ, would have helped immensely.
IITs in Delhi and Mumbai are setting up research parks within the campus that should help in a small way. “India is the biggest graveyard of prototypes that have failed to commercialise themselves,” says prof Anil Gupta, executive vice-chairman, National Innovation Foundation.
Meanwhile, Singh, Pandey, Elangovan and Hasnain have set up a startup called Valetude Primus Healthcare (VPH) to spearhead the job of manufacturing and marketing the product. The team has been scouting for suppliers in nooks and corners of Chandni Chowk to figure how to take forward the manufacturing plans.
Many more challenges await them. The product itself has to be refined and needs to get the regulator’s approval before commercial deployment next year. Competitive intensity is daunting.
With deep pockets, backed by governments in different countries, often large companies globally resort to predatory pricing to kill fledgling competition. VPH is bracing for it.
Predatory pricing can wipe out a generation of innovation-based companies. The Indian government is waking up to some of these issues. Swarup says the government is exploring ways it could assist in solving some of the problems like funding, mentoring, manufacturing and clinical validation. “A researcher from the academia is working to build an enterprise in risky areas. We want to help as much as we can,” says Swarup.
Meanwhile, Elangovan remains upbeat. “We have to build hard core technology, get patents and build interesting products and commercial solutions around it,” he says. If that happens, success, glory and money will come automatically for these scientists.
Also, that’s the only way research and researchers can become an aspirational class in India

