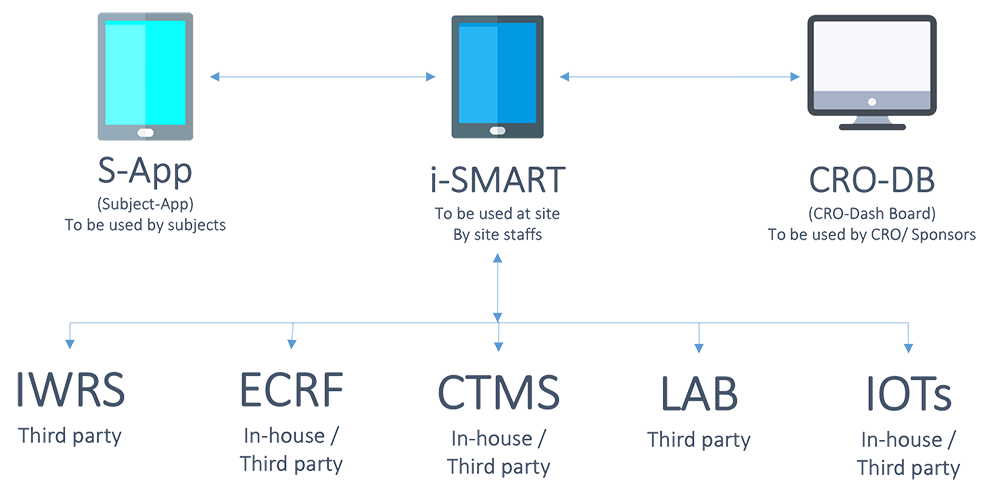
iSMART is the first integrated, dynamic, flexible solution to remotely control study conduct and data recording at site level which is developed on the concepts like e-Source, e-ICF, Digital protocol and having its control system as the crux to give the ultimate benefit by significantly reducing cost and time of drug development.
Key Features
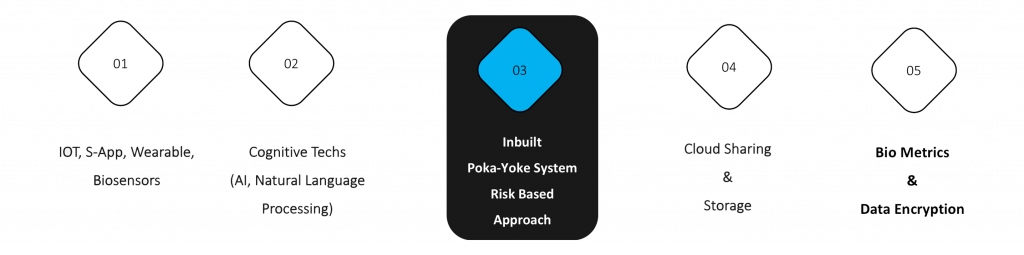
Goal: The goal of iSMART is to innovate the clinical trial conduct and recording of data at site level by achieving the following.
- To digitalize all possible aspects of Clinical Trial conduct at site level by implementing key components of e-Source, e-ICF and Digital Protocol.
- To get access to trial data in actual real time by integrating all data source to iSMART and transfer the same to analytical team in real time with triggers installed to address risks more quickly than ever.
- To have a mature control system by implementing poka-yoke concept to avoid all possible protocol deviations in order to avoid wastage of time and cost in drug development.
- To reduce the time and cost spent on training trial sites by 50% by implementing 100% iSMART driven trial conduct at site.
- To reduce the time and cost of study monitoring by 50% by having strong control system and self-risk identification, analysis and mitigation system.
What is iSmart?
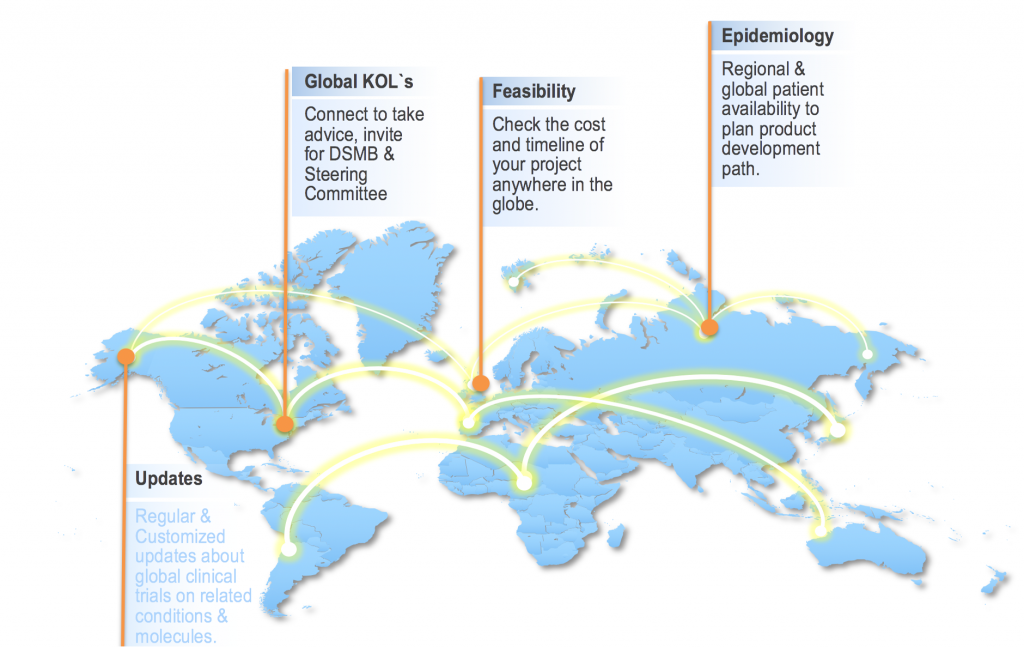
iPET (Innovative Patient Enrollment Tool)
i-PET-Innovative Patient Enrollment Tool is a cloud based solution to revolutionize feasibility, study conduct and patient enrollment. i-PET facilitates Global connectivity to achieve fastest ever feasibility survey/study across the globe, and it enhances site performance with respect to recruitment by cross references and its unique patient enrollment process.
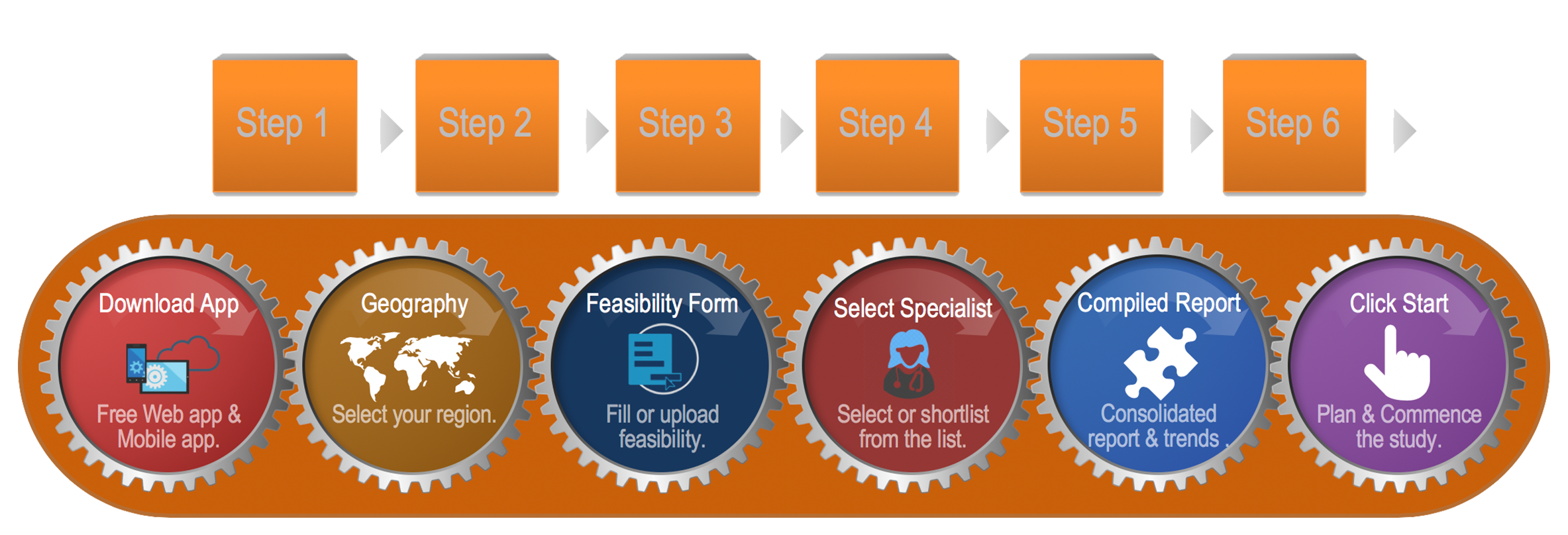
How to use the iPET?